So I want to start a series of posts that will focus mostly on lasers, what they are, how they work, what are the most important elements, etc… It can still evolve.
Some prerequisites first: To fully understand what I will write, here, it definitely helps to be familiar with a few concepts and bases from electromagnetism and optics, obviously. I’ll try to explain these concepts along the way of course. Nevertheless, even if you don’t have the necessary background, keep reading. I’ll try to keep it simple and limit the mathematics to a minimum.
It’s no surprise that lasers have become widespread nowadays. They are used in printers, display, range finders, communication, industrial applications for cutting, welding, engraving, directing lightning, and many more…
How does a laser work? what is the difference between a laser source and a lamp or a LED? I’ll try to cover these in this first post.
Light on
Some basic definition first: what is light exactly? There are many ways to see light (pun intended). For this discussion, it is better to see light as a packet of energy, like a small blast, that is emitted in a specific direction. Usually, physicist call that packet of energy a photon. Without entering too much in the details here, a photon can be considered as a particulate or a electromagnetic wave. The most important part is that this photon has a given energy, which is related to its wavelength (\lambda) with the following relation:
E = \dfrac{hc}{\lambda}
From this very important equation, the idea to keep is that, because h (Plank’s constant) and c (speed of light) are constants, the energy is inversely proportional to the wavelength of the photon. A single ultra-violet photon carries more energy than a single infra-red photon for instance. Another way to see it is that UV photons are so energetic that they interact with matter very quickly, and so are absorbed quite fast. Visible and IR will go deeper in general.
Emission spectrum
Now, let’s talk about light sources. A light source is a source of photons. Photons are generated by several physical (or chemical) processes. One of these process is simply heat. If you have any sort of matter with any amount of heat, this matter is going to emit photons.
A heated material is going to emit photons according to an emission spectrum. This emission spectrum depends on the temperature and the material. Why does it depends on the temperature? When the material is cold, not a lot of energy can be converted into photons, so on average, the wavelength is going to be in the far infra-red. If the material gets heated more, more energy can be converted into photons, and the wavelength is going to be more toward red, going to visible and eventually UV. Eventually, one can get a rough idea of the distribution of the emitted photons by a heated material by looking at the black body spectrum.
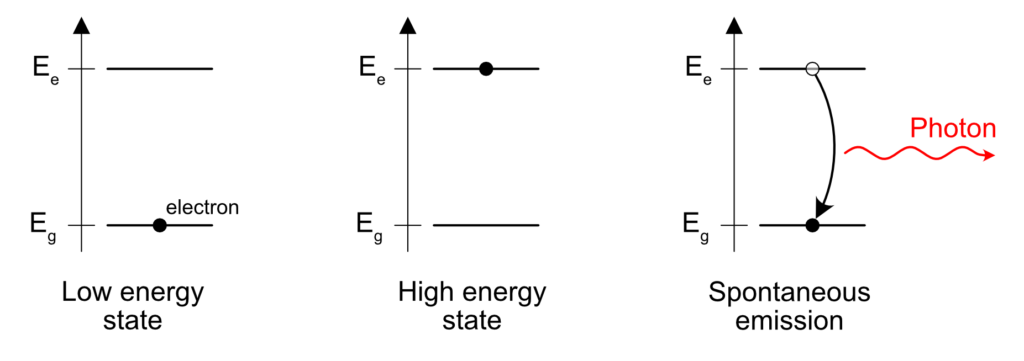
Now, why does the spectrum depends on the material? Well, one can assume that photons are mostly emitted by electrons. When an atom is in an excited state because of the heat, the electrons in that atom have a given amount of energy and eventually, this energy gets released when an electron goes from a high energy state to a lower energy state. Excited electrons don’t tend to stay that way and lose the excess energy to go back to their rest state. The release of energy can give birth to a photon carrying the exact energy between the initial and final state of the electron.
These difference between initial and final state of the electron depends on the atom we have, because of the electronic structure of the atom that is their fingerprints. A good example is when iron is heated, it starts to glow red and eventually turn white before melting. Iron’s emission spectrum looks like the following image:
Heated hydrogen, a simple, light atom, will have a simpler emission spectrum like the following image:
The act of release of the photon is spontaneous, based on a random process. An electron will stay in the high energy level a given amount of time, and the release of the energy depend on some probabilistic rule. Usually, these times are very short: I’m talking about picoseconds, nanoseconds and eventually microseconds. In the case of phosphorescence, the lifetime is actually really long, I’m talking milliseconds to above.
Light sources using a heated source used to be everywhere: light bulbs for instance were the main source of light at some point. They are now being replaced by LEDs. LEDs don’t use heat to generate light, they use a principle called electroluminescence. Neon on the other hand rely on ionizing a gas.
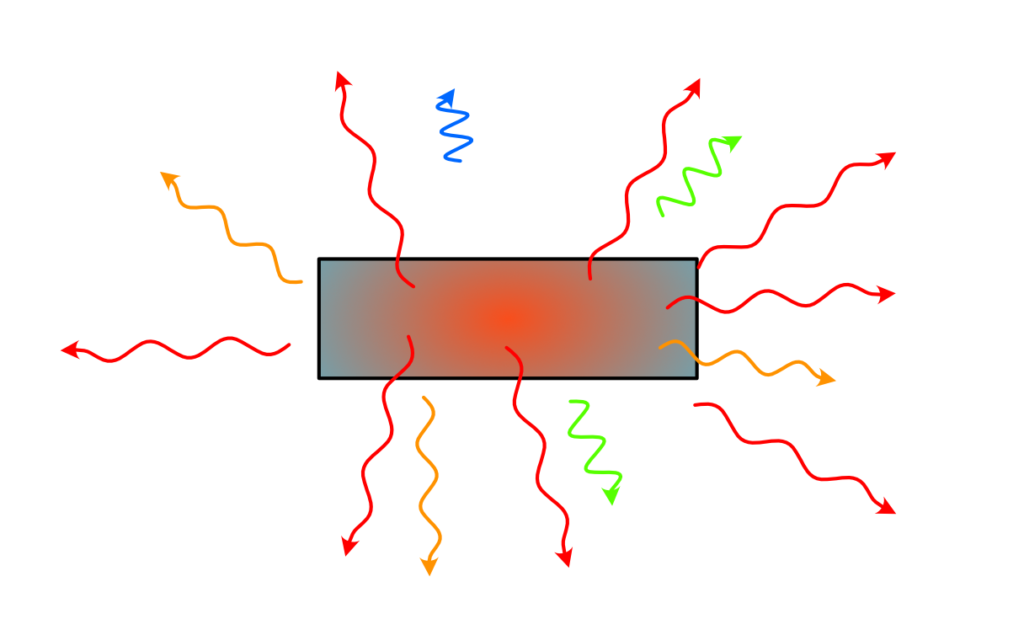
One important point to remember here is that these sources of light emit photons in a large wavelength range. Because the emission is not well controlled and is happening randomly, it doesn’t have a precise direction, nor a precise timing of emission. We therefore have a source of light that is broadband, i.e. emitting in a large spectrum, but also isotropic, i.e. emitting in all direction. Not forgetting that this source is continuous, i.e. it emits photons in a continuous manner, without pulsing, without making packets of photons or whatever.
Some history of the origins…
The idea of the laser is old. One major element allowed the laser to be discovered: the existence of spontaneous and stimulated emission when an atom is excited. Who discovered it? Unsurprisingly, it is Einstein in 1917. Yes, technically, the laser is an 100 year old idea.
But between this idea and the actual building of the first laser, a lot of time passed. In 1951, Townes had the idea of a maser, a microwave emission device based on the same principles of the laser. Townes built a working maser in 1954. It is often seen as the precursor of the first laser.
The first laser was built by Maiman in 1960, when he was working in the Hughes Research Laboratory: a ruby laser at 694 nm.
But I notice: I talk a lot about laser and I have not even defined why we call them that way.
L.A.S.E.R.
Laser is actually an acronym. Not a lot of people know about it outside of the photonics and optics community. It stands for Light Amplification by Stimulated Emission of Radiation.
Light Amplification: we have more and more light.
Stimulated Emission of Radiation: we have here the basic principle from Einstein, the stimulated emission, which is at the core of every laser device and at the source of its characteristics compared to a “regular” light source.
Let’s dig a little deeper in the subject….
Stimulated Emission
Stimulated emission is sort of opposed to spontaneous emission.
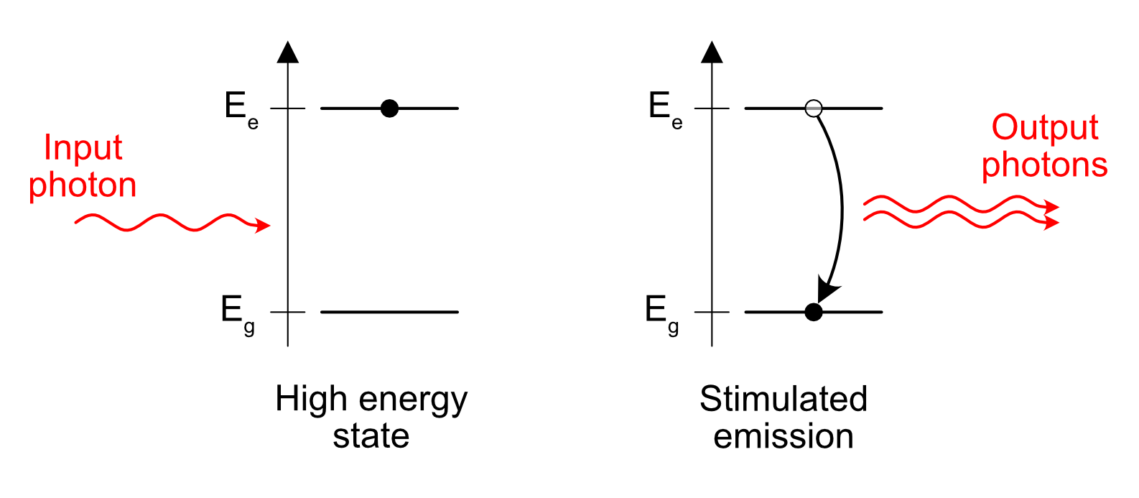
Take an excited atom. Some electron is “energized” above its natural level and needs to emit this amount of excess energy somehow. When does it happen? Like I wrote before, in the case of spontaneous emission, the release of the excess energy is “spontaneous”, that is to say it happens at pseudo-random.
But something interesting happens if an excited atom is standing close to a passing photon with the right energy. One can see photons as a friendly bunch of particles. To put it in more scientific terms, photons are bosons. Bosons tend to group easily and the presence of a photon near an excited atom is enough to trigger the release of a similar photon, in the same direction and phase than the passing, already existing photon. Sort of like a perfect copy. This is what stimulated emission means. The emission of a photon is stimulated by the presence of another similar photon already in the vicinity of the excited atom.
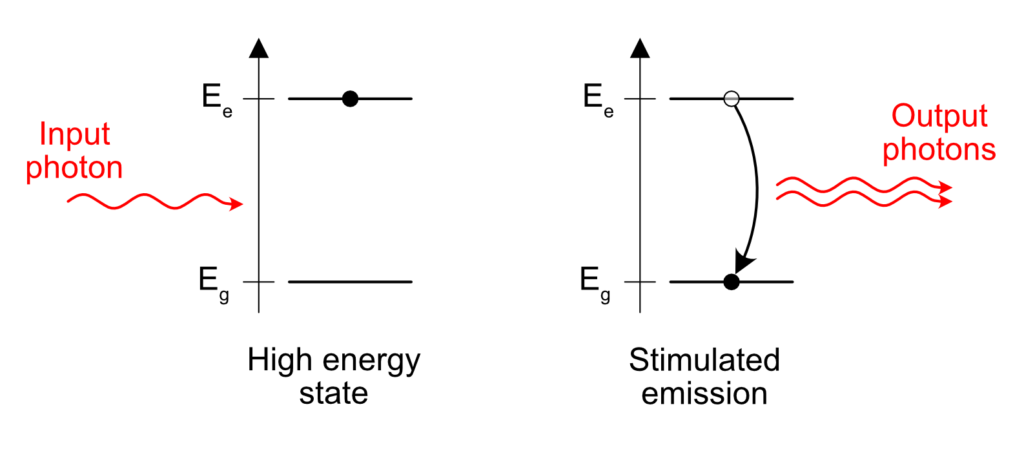
This is the core principle of a laser. If you understood this, you understood 70% of what lasers are all about.
Amplification
So we know how to transform 1 photon into 2 photons identical to the first one… This is huge. What next? We need to amplify the effect, turn those 2 photons into 4, then 8 then 16 etc…
In a laser, this amplification is performed inside what is called an gain medium. It is usually a crystal, but can be a gas, a liquid, etc… This gain medium usually gives its name to the laser. So a Nd:YAG laser is a laser whose gain medium is a crystal of yttrium aluminium garnet (YAG) doped with Neodymium (Nd). It is composed of atoms that have the ability to become excited by a process called pumping, and most importantly, have long spontaneous emission time. I’ll talk about that in another post because there is a lot of physics involved here.
Pumping can be performed electrically, by sending electrons to the medium, or usually… with some other light! Flash lights, very powerful are used to send energy to the medium and excite the atoms. Eventually, some atoms are going to spontaneously emit a photon at the right wavelength and this photon is going to trigger the stimulated emission and make the material “lase”.
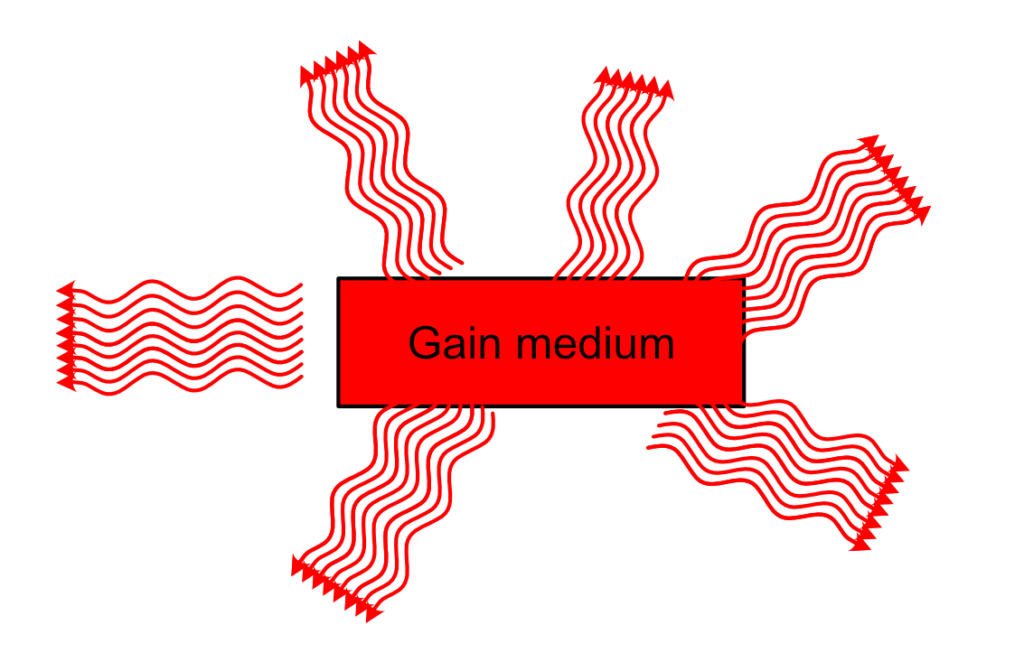
From this, you can technically have a functioning laser. One caveat is that the light you are going to receive out of this system, even if it is technically laser light, won’t be fully coherent and unidirectional. The detail you might have miss is that the triggering photon comes from spontaneous emission. This spontaneous emission is random in direction and phase, and once the train of photons that will be stimulated by this photon leaves the medium, there is absolutely no reason why the next spontaneous photon be in phase, in the same direction as the first one.
We need to filter the photons and recycle them…
Laser cavity
This filtering and recycling is performed quite simply. When the train of photons leaves the medium, we need to catch them and send them back in the medium. In other words, we need a mirror. Actually, we need two mirrors to create a real trap.
This way, as you can imagine, a train of photon created by a single spontaneously emitted photon will travel forever between those two mirrors, passing each time through the medium and triggering another wave of stimulated emission, creating ever more photons with the same phase and direction. Perfect.
Again, the physics behind this is much more complex, but this is the general, simplified idea. I’ll spend a full post later explaining the cavities another week.
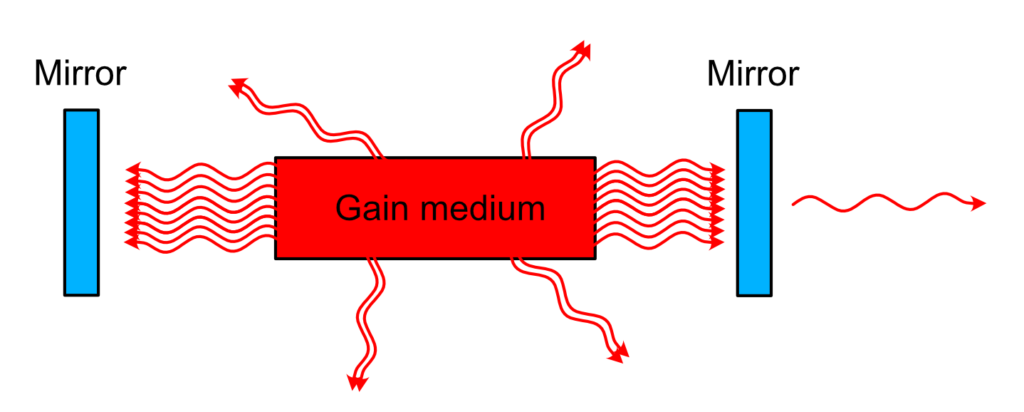
Now, how to let some light out? One of the cavity mirror is not perfect and let a few percent leak outside. This few percent is going to be the laser beam, ready to be used for whatever application you want.
This is it, this is how you get laser light from scratch, explained, I hope, relatively easily with minimum equations and complications.
That was a long one. Re-reading this, I think it covers the surface for someone to understand the basics behind lasers, but I will dig even deeper in some core concepts I haven’t even mentioned here, like population inversion, gain, mode locking, etc… in other posts. In other words, it’s going to be really interesting. It’s also really nice for me to review and refresh all these topics.
Sources and references
[2] Laser fundamentals by Silfvast